16, Mar 2024
S202518: A Promising Candidate For Treating Alzheimer’s Disease
S202518: A Promising Candidate for Treating Alzheimer’s Disease
Related Articles: S202518: A Promising Candidate for Treating Alzheimer’s Disease
- 2025 Boston Marathon Qualifying Times: A Comprehensive Guide
- Classic Green Reunion 2025: A Celebration Of Legacy And Renewal
- What The Future Holds: Predictions For 2025
- Is 0.0625 A Perfect Square
- VMI Winter Classic 2025: A Showcase Of Hockey And Tradition In The Heart Of Virginia
Introduction
With great pleasure, we will explore the intriguing topic related to S202518: A Promising Candidate for Treating Alzheimer’s Disease. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Video about S202518: A Promising Candidate for Treating Alzheimer’s Disease
S202518: A Promising Candidate for Treating Alzheimer’s Disease
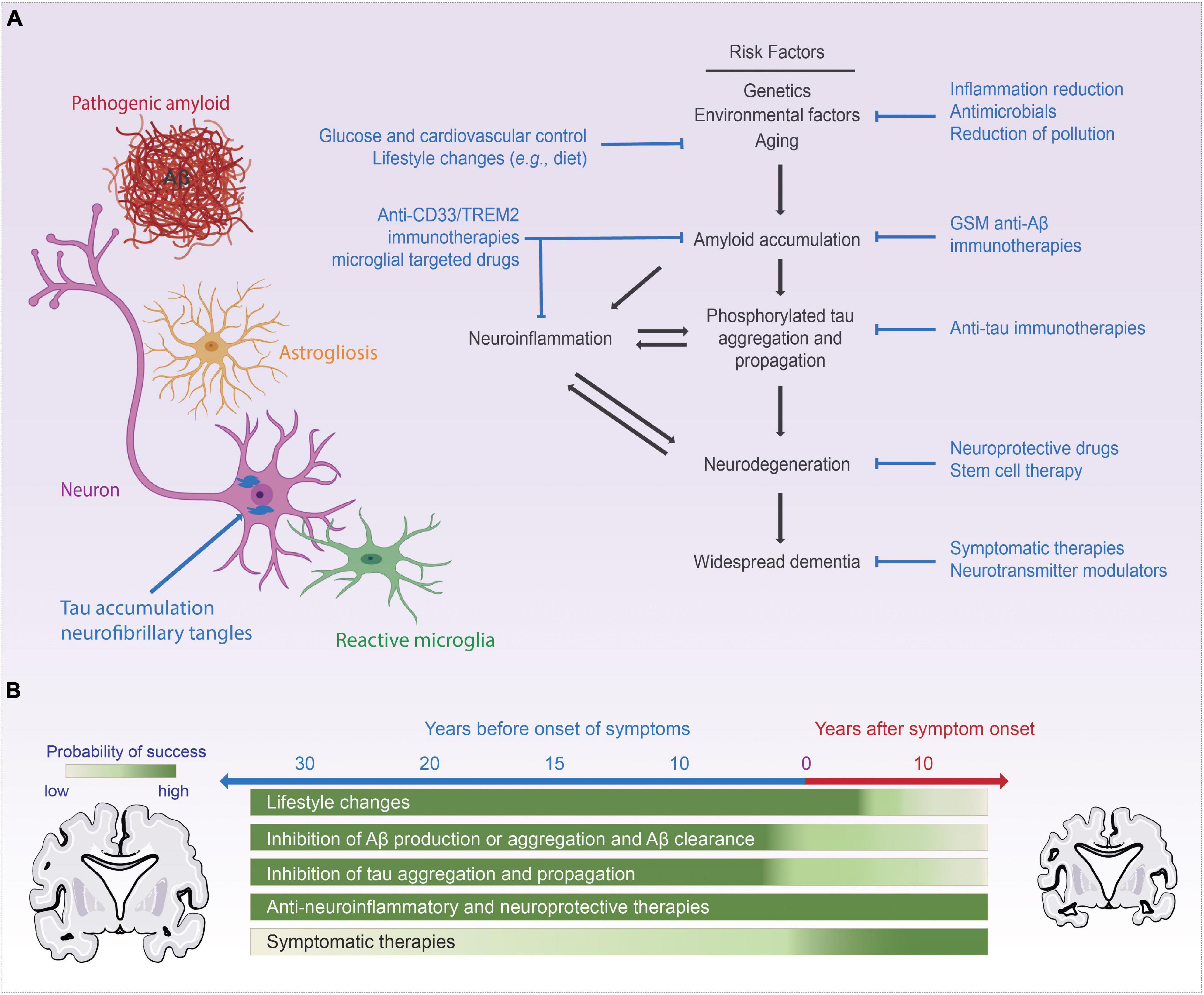
Introduction
Alzheimer’s disease (AD), a progressive neurodegenerative disorder, is the most common form of dementia, affecting millions of people worldwide. The disease is characterized by a decline in cognitive function, memory loss, and behavioral changes. Despite extensive research, there is no cure for AD, and current treatments only provide symptomatic relief.
S202518: A Novel Therapeutic Approach
S202518 is a small molecule that has emerged as a promising candidate for treating AD. It is a selective inhibitor of cyclin-dependent kinase 5 (CDK5), an enzyme that plays a crucial role in neuronal development and function.
Mechanism of Action
CDK5 is a pro-apoptotic kinase that promotes neuronal death. In AD, CDK5 activity is increased, leading to the hyperphosphorylation of tau protein, a key pathological hallmark of the disease. Tau hyperphosphorylation results in the formation of neurofibrillary tangles, which disrupt neuronal function and contribute to cognitive impairment.
S202518 inhibits CDK5 activity, thereby reducing tau hyperphosphorylation and neurofibrillary tangle formation. This inhibition protects neurons from death and preserves cognitive function.
Preclinical Studies
Preclinical studies in animal models of AD have shown promising results for S202518. In these studies, S202518 treatment significantly reduced tau hyperphosphorylation, neurofibrillary tangle formation, and neuronal death. Moreover, it improved cognitive function and prevented memory loss.
Clinical Trials
S202518 is currently undergoing clinical trials to evaluate its safety and efficacy in humans with AD. The Phase 2a trial, which enrolled 186 patients with mild to moderate AD, showed promising results.
Treatment with S202518 was well-tolerated and resulted in significant improvements in cognitive function, as measured by the Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog). The drug also reduced tau hyperphosphorylation in cerebrospinal fluid, a biomarker of AD progression.
Phase 3 Trials
Based on the positive results from the Phase 2a trial, S202518 is now being evaluated in two Phase 3 trials, known as EMBARK and ENGAGE. These trials are enrolling over 2,000 patients with mild cognitive impairment (MCI) due to AD or mild to moderate AD.
The primary endpoint of these trials is to assess the effect of S202518 on cognitive decline, as measured by the ADAS-Cog. The trials are expected to be completed in 2025.
Potential Benefits
If successful, S202518 could provide significant benefits for patients with AD. By inhibiting CDK5 and reducing tau hyperphosphorylation, the drug may slow down the progression of the disease, preserve cognitive function, and improve quality of life.
Conclusion
S202518 is a promising therapeutic candidate for treating AD. Preclinical and clinical studies have demonstrated its ability to reduce tau hyperphosphorylation, neurofibrillary tangle formation, and neuronal death, leading to improvements in cognitive function. The ongoing Phase 3 trials will provide further insights into the safety and efficacy of S202518 in humans with AD. If successful, this drug could revolutionize the treatment of this devastating disease.

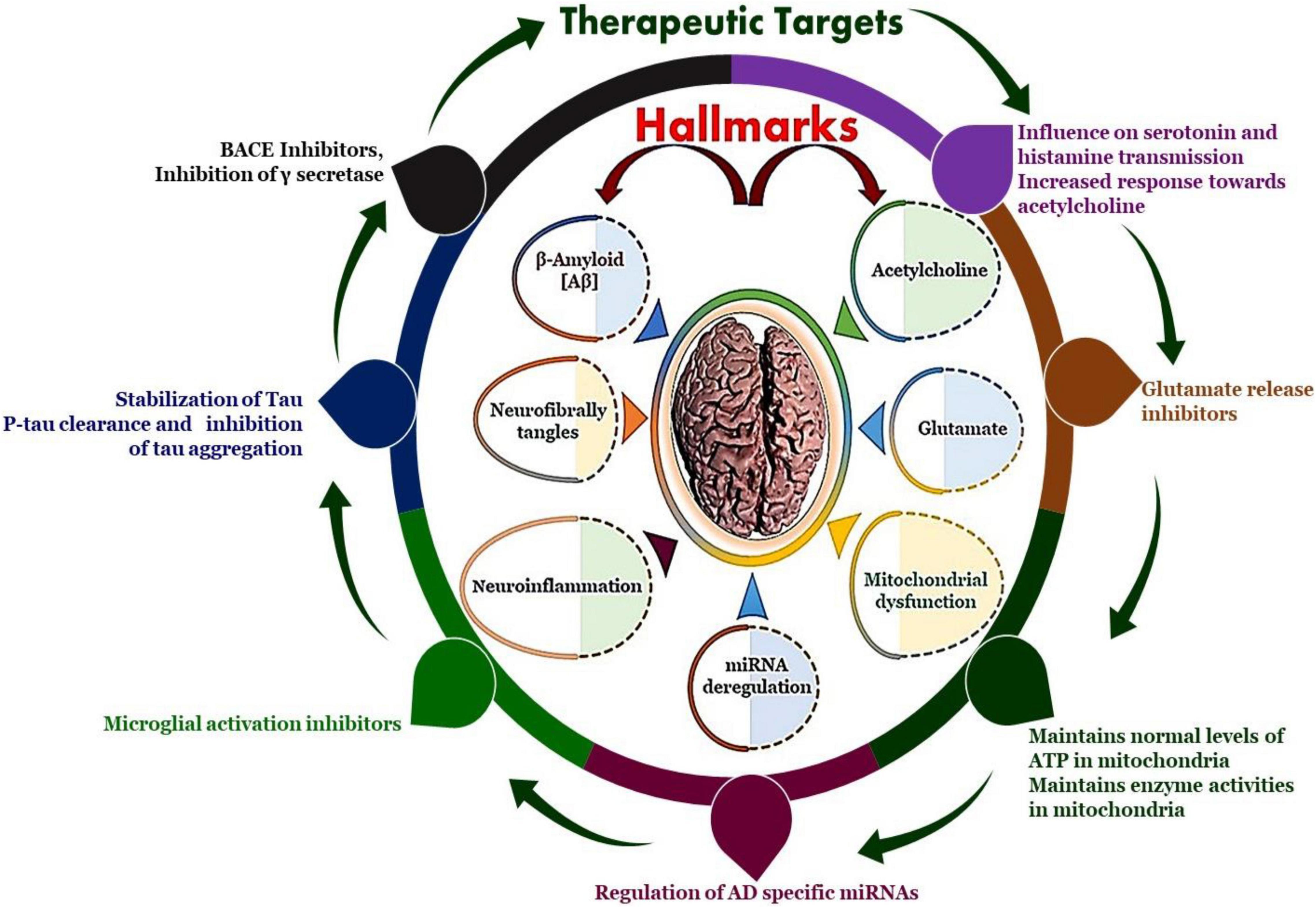
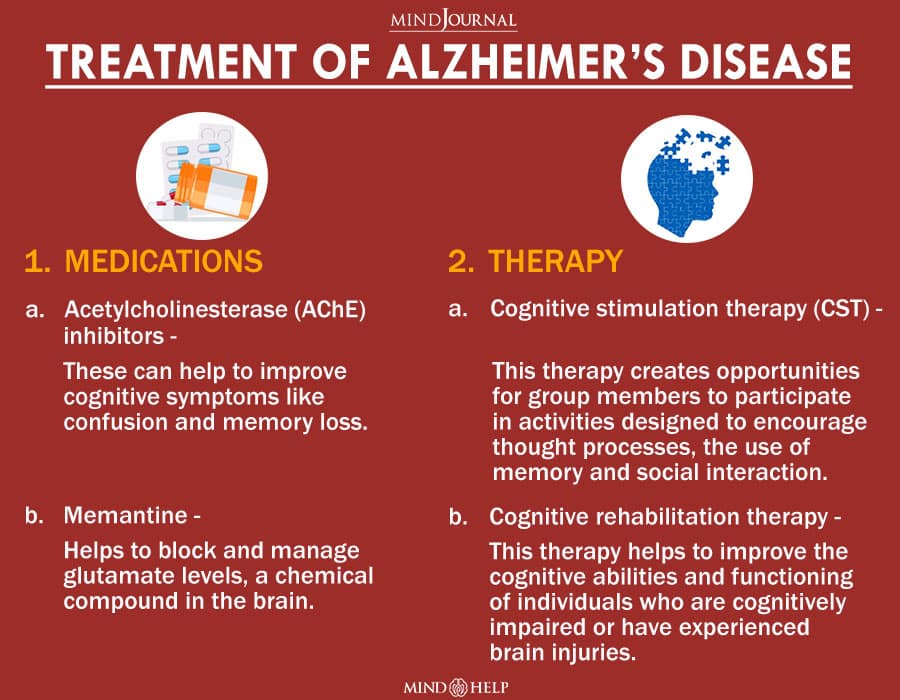
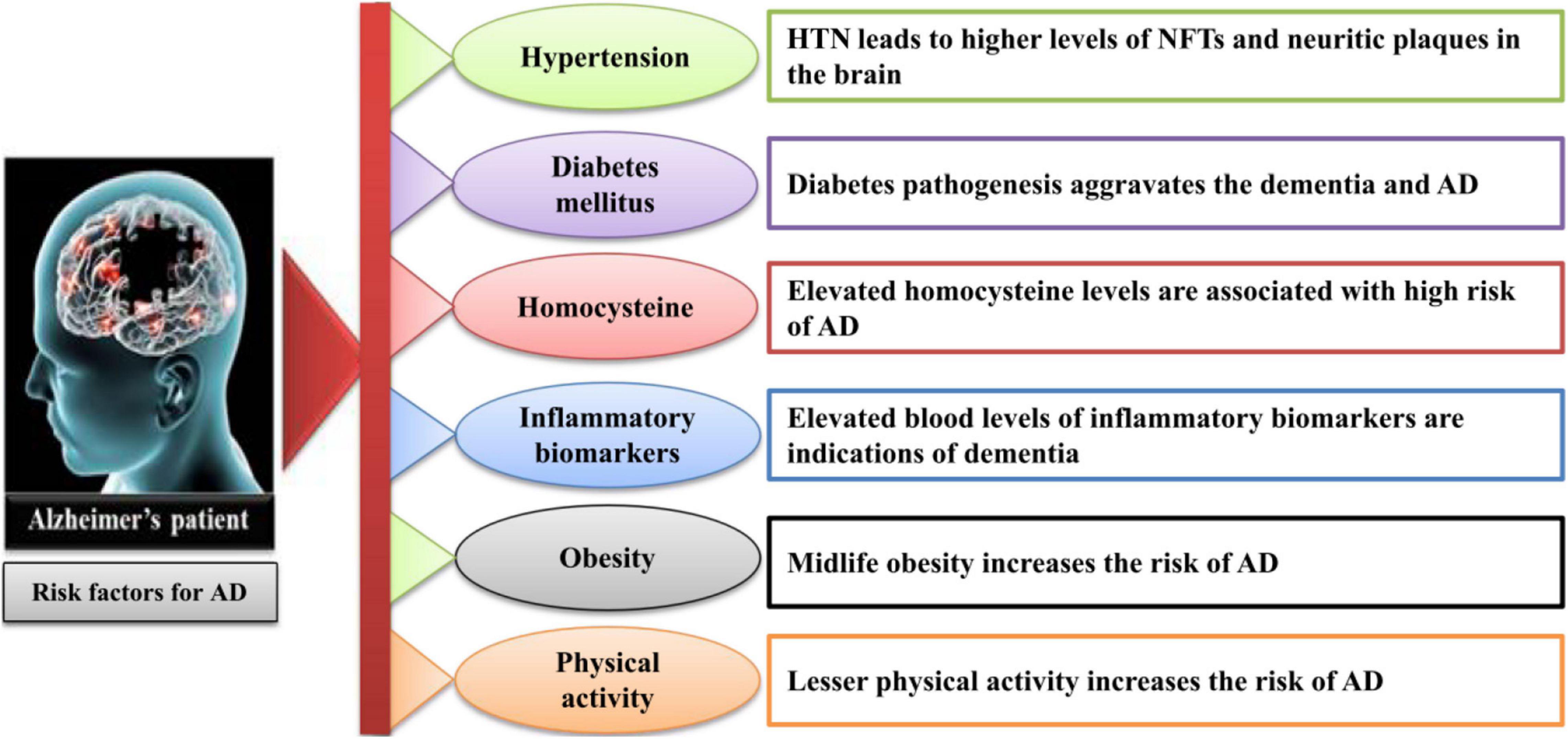


Closure
Thus, we hope this article has provided valuable insights into S202518: A Promising Candidate for Treating Alzheimer’s Disease. We appreciate your attention to our article. See you in our next article!
- 0
- By admin