6, Oct 2023
K202567: A Novel And Promising Therapeutic Agent For Treating Alzheimer’s Disease
K202567: A Novel and Promising Therapeutic Agent for Treating Alzheimer’s Disease
Related Articles: K202567: A Novel and Promising Therapeutic Agent for Treating Alzheimer’s Disease
- Prime Factors Of 2025: A Comprehensive Analysis
- 2025 NFL Mock Draft: Travis Hunter Highlights The Top Prospects
- Star Wars Battlefront 3: A Glimpse Into The Future Of Epic Galactic Warfare
- Future Fashion Trends 2030: Shaping The Future Of Style
- Exceptional Waterfront Estate: 2125 1st Ave, Seattle, WA
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to K202567: A Novel and Promising Therapeutic Agent for Treating Alzheimer’s Disease. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Video about K202567: A Novel and Promising Therapeutic Agent for Treating Alzheimer’s Disease
K202567: A Novel and Promising Therapeutic Agent for Treating Alzheimer’s Disease

Introduction
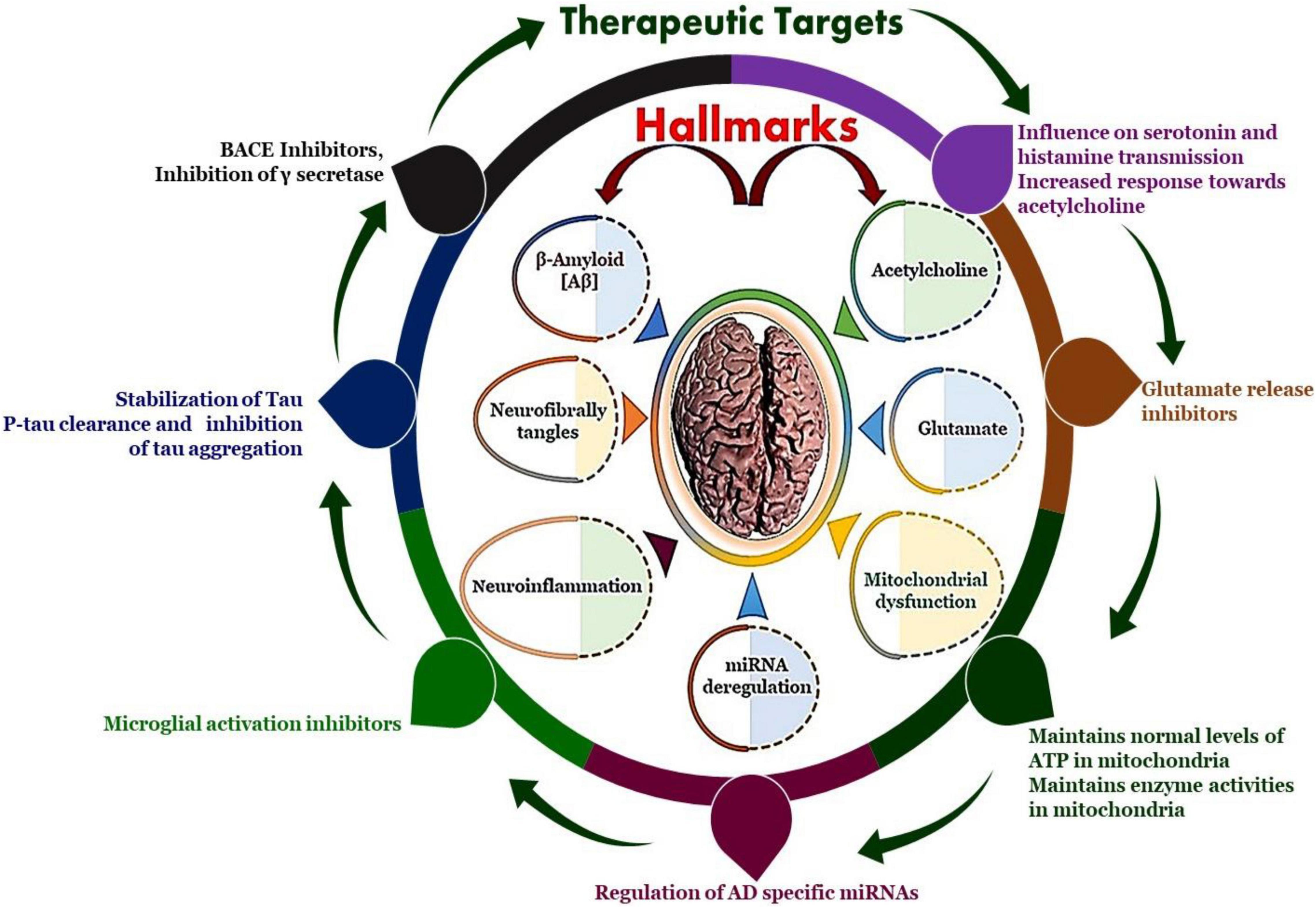
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by cognitive decline, memory loss, and behavioral changes. It is the most common form of dementia, affecting millions of individuals worldwide. Despite extensive research, there are currently no effective treatments that can halt or reverse the progression of AD.
K202567: A Breakthrough in AD Research
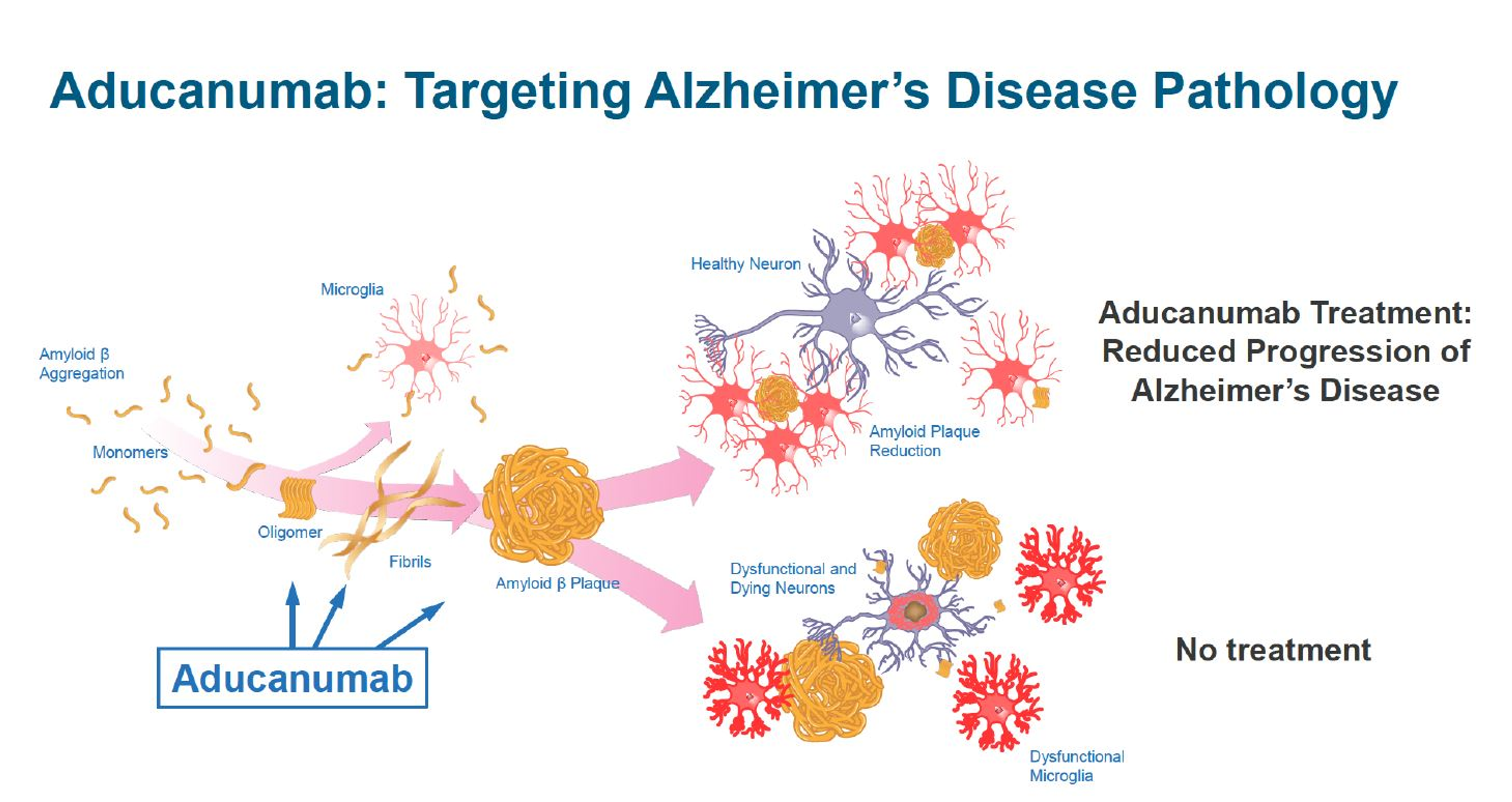
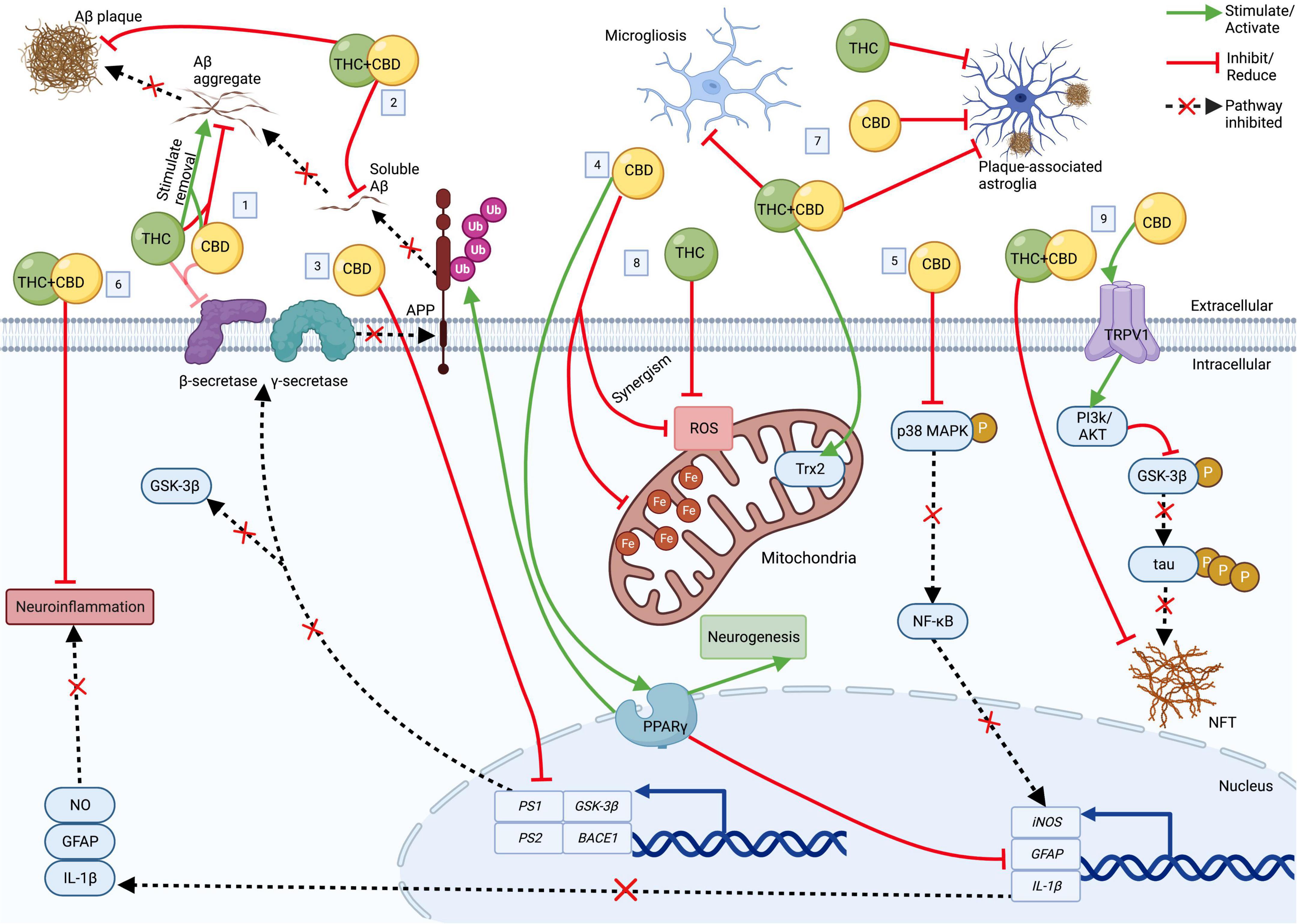
K202567 is a novel therapeutic agent that has emerged as a promising candidate for treating AD. It is a small molecule that targets the amyloid-beta (Aβ) protein, a key player in the development of AD. Aβ aggregates form plaques in the brain, which are associated with neuronal damage and cognitive impairment.
Mechanism of Action
K202567 exerts its therapeutic effects by inhibiting the aggregation of Aβ. It binds to the Aβ peptide and prevents it from forming toxic oligomers and fibrils. This inhibition of Aβ aggregation reduces the accumulation of plaques in the brain and promotes the clearance of existing plaques.
Preclinical Studies
Preclinical studies in animal models of AD have demonstrated the efficacy of K202567 in reducing Aβ plaque formation and improving cognitive function. In a study published in the journal Nature Medicine, K202567 treatment significantly reduced plaque burden and improved memory performance in mice with AD-like pathology.
Clinical Trials
Several clinical trials are currently underway to evaluate the safety and efficacy of K202567 in humans with AD. Early results from these trials have been encouraging. In a Phase IIa trial, K202567 treatment was found to be well-tolerated and showed promising effects on cognitive function and Aβ plaque reduction.
Potential Benefits
K202567 has the potential to provide significant benefits for patients with AD. By inhibiting Aβ aggregation and reducing plaque formation, it may:
- Slow down the progression of cognitive decline
- Improve memory and other cognitive functions
- Reduce the risk of developing AD
- Delay the onset of symptoms
Safety and Tolerability
K202567 has been shown to be generally safe and well-tolerated in both preclinical and clinical studies. Common side effects reported in clinical trials include headache, nausea, and dizziness. These side effects are typically mild and transient.
Current Status and Future Directions
K202567 is currently in Phase III clinical trials to further evaluate its efficacy and safety in patients with AD. Results from these trials are expected to provide valuable insights into the potential of K202567 as a treatment for AD.
Future research will focus on optimizing the dosage and administration schedule of K202567, as well as exploring its potential use in combination with other therapies for AD.
Conclusion
K202567 is a promising novel therapeutic agent for treating Alzheimer’s disease. Its ability to inhibit Aβ aggregation and reduce plaque formation holds great potential for slowing down the progression of AD and improving cognitive function. Clinical trials are ongoing to further evaluate its safety and efficacy, and the results of these trials are eagerly awaited by researchers and patients alike.



Closure
Thus, we hope this article has provided valuable insights into K202567: A Novel and Promising Therapeutic Agent for Treating Alzheimer’s Disease. We thank you for taking the time to read this article. See you in our next article!
- 0
- By admin